Anomalous ‘homology’ refutes evolution
The introductory article ‘Homology - for or against evolution?’ summarised the concept of homology; and it outlined how, although homology is generally presented as evidence for evolution, in fact there are some important exceptions which not only remove homology as support for evolution but seriously undermine it. Indeed, examples of non-homology present a cogent case against both common descent and macroevolution.
This article examines the issue further - first presenting more examples, in greater detail, and then considering the evolutionary response (or lack of it!) to this highly significant evidence.
Homology and evolution
Historically, homology was simply the term used to describe similarities of biological structure. For example, Richard Owen[1] regarded homologous structures as characteristic features of archetypes or types of body plan.
The theory of evolution seemed to provide a convincing explanation for homologies in terms of phylogeny: homologous organs in different groups of species are similar because they have been inherited - with some degree of modification - from a common ancestor. Indeed, this evolutionary explanation has become so widely accepted that it now defines homology as referring to those organs which have been derived from the same structure in a common ancestor.
Homology. Originally, correspondence in fundamental structure of an organ, part, etc. Now chiefly, correspondence in evolutionary origin (of organs, parts, etc.).[2]
Criteria for homology
a. Equivalent embryological source
To be consistent with the evolutionary interpretation of homology - that homologous features are essentially the same structure, but adapted differently in different organisms - it necessarily follows that homologous structures must have developed from equivalent tissues in the embryo.
b. Comparable developmental processes
Further, it has long been recognised that early stages of embryological development should be conserved; because so much later development depends on it, changes to early development will lead to detrimental deformities rather than something useful. And this view is reinforced as we learn more about how embryos develop – the complex interplay of many genes, transcription factors and inducers, and the molecular bases for cell differentiation and movement that are key to tissue formation. Hence it follows that for homologous organs or tissues the developmental processes should be comparable.
A necessary component of homology is the sharing of a common developmental pathway. Homologues must, to some extent, follow similar processes of differentiation which, one infers, depends on the same batteries of (regulatory and structural) genes [...] homology is based on the sharing of pathways of development which are controlled by genealogically related genes.[3]
-----
For any departure from these criteria there must be a viable evolutionary route, i.e. attainable through small random steps, each with a realistic chance of happening, and conferring an advantage that natural selection can operate on. In fact it is because there is a prima facie case, accepted by evolutionists, that such changes could not take place, that these are criteria.[4]
Homology – the inconvenient truth
Although most evolutionary texts convey a consistent and hence persuasive picture of homology, there are in fact many substantial anomalies. In particular, as we discover more of how tissues are formed embryologically, increasing doubt is cast on much of the homology that has been perceived for so long at the morphological level. There are many exceptions: where organs generally considered to be homologous are not formed by equivalent developmental processes, or even arise from different embryological tissues. Given the frequent use of the vertebrate skeleton to illustrate homology, here I will focus on the non-homology of vertebrae from different vertebrate classes.[5]
Vertebrae
‘Homology - evidence for or against evolution?’ mentioned that despite the obvious similarities of the vertebrate backbone, which readily suggests a common origin, in fact there are substantial differences in the embryological formation of vertebrae. This clearly undermines their supposed homology - at least in an evolutionary sense of having derived from a common ancestor. Here I’ll look at these differences in some detail. Kardong[6] provides a useful overview.
Tetrapods (e.g. reptiles, birds, mammals)
Early in the development of a tetrapod embryo a neural tube forms along the embryo’s back – this will develop into the spinal cord. Running alongside (ventral) this is the notochord which is a supporting structure in the early embryo and is the focus for formation of the spinal column. However, in tetrapods, the vertebrae do not form from the notochord itself; rather, these develop from somites which arise in pairs, either side of and along the length of the neural tube. So at an early stage of development we have the situation illustrated in Figure 1a.
In most tetrapods the cells of the somites differentiate into three distinct layers: on the outside is dermatome which will develop into connective tissue, then myotome which will develop into muscle, and innermost is sclerotome which develops into the vertebrae.
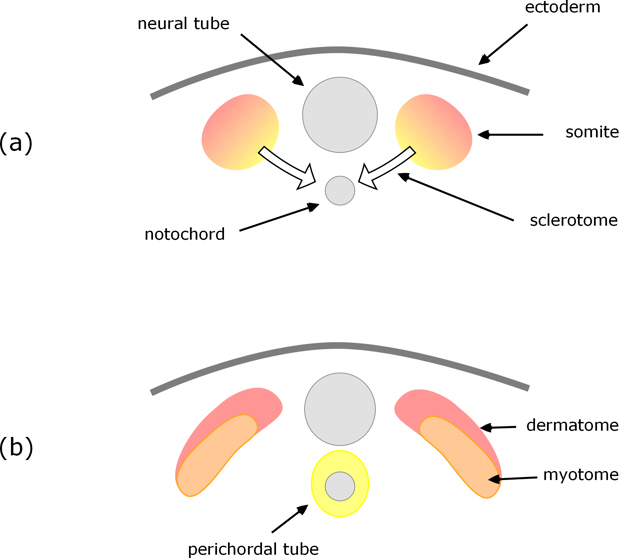
Figure 1. Cross-section through a pair of somites as they differentiate into dermatome, myotome, and sclerotome which migrates to surround the notochord.
The sclerotome cells grow and migrate towards the notochord, cells from both sides of the neural tube, combining and surrounding the notochord to form what is called the perichordal tube (Fig. 1b); this then develops into cartilaginous vertebrae which subsequently ossify to give the bony vertebrae.
The notochord all but disappears: some contributes to the intervertebral discs, but none to the vertebrae themselves.
Resegmentation
A notable and somewhat unexpected feature of vertebrae formation in tetrapods is resegmentation. As the sclerotome cells move towards and envelop the notochord, the posterior (tail-ward) end of one combines with the anterior (head-ward) end of the adjacent one (Fig. 2b), and at the same time an intervertebral disc forms within each perichordal ring (Fig. 2c). The result is that each vertebra is formed from the posterior half of one perichordal ring and the anterior half of the adjacent one. That is, the vertebrae do not arise from the somites one-for-one, but each is derived from adjacent halves of neighbouring somites.
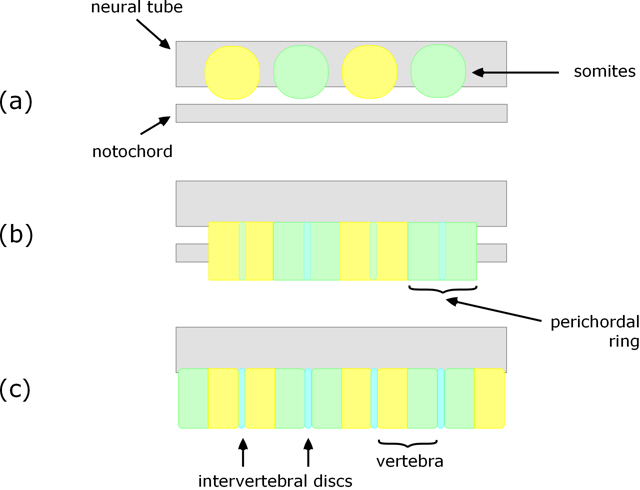
Figure 2. Longitudinal section (vertical) showing initial position of somites (a), perichordal tube arising from sclerotome surrounding the notochord (b), and resulting vertebrae (c).
Cartilaginous (e.g. shark) and some bony fish
Formation of vertebrae is somewhat different in cartilaginous fish such as sharks and many so-called ‘primitive’ bony fish such as sturgeons. In these sclerotome cells migrate towards the notochord, but rather than surrounding it they form into blocks of cartilaginous tissue called arcualia. Typically, each pair of somites produces four pairs of arcualia, with one of each pair on either side of the neural cord / notochord. The arcualia then transform into a vertebra, with each of the arcualia destined to become a specific part of it (see Fig. 3).
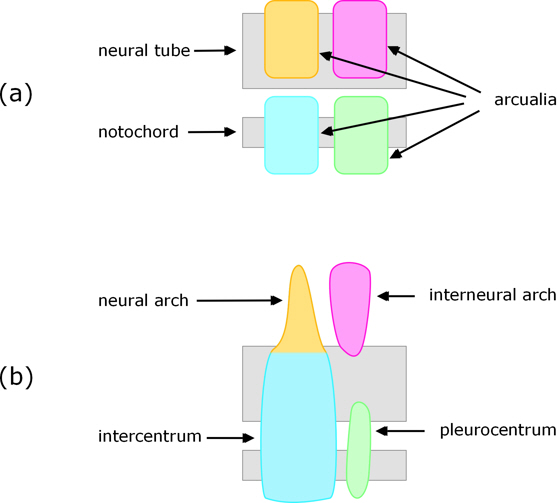
Figure 3. View of arcualia from one side (a) and resulting parts of the vertebra (b).
The differences from the tetrapods are so substantial that Earnest Williams[7] concluded:
Thus there seems to be no simple way of comparing shark vertebrae and tetrapod vertebrae; there exists indeed a reasonable doubt that they are built to the same plan.
Teleosts (most fish)
A completely different development route is used in teleosts. In these the innermost part of the vertebrae arises directly from the notochord (i.e. not from sclerotomes, although these contribute to outer parts of the vertebrae), see Figure 4. Nordvick[8] gives a recent and detailed account.
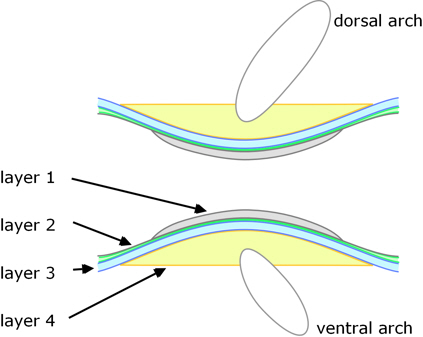
Figure 4. Longitudinal section though a teleost vertebra.
There are four distinct layers:
1. The development of the vertebral bodies is initiated within the notochord: within its sheath cartilaginous elements arise and mineralise to form the inner layer of each vertebra.
2. The notochord is then encased by a layer of collagen fibres; this extends the whole length of the notochord, becoming thickest between the vertebrae where it contributes to the intervertebral ligaments, whereas the vertebral regions become mineralised.
3. Outside of this, a layer of dense bone is deposited.
As the preceding steps proceed, undifferentiated cells (mesenchyme) on top and bottom of the notochord (dorsal and ventral) begin to form cartilaginous arch centres.
4. Sclerotome-derived cells deposit bone onto the outside of the inner three layers (and enclosing the arch centres).
Features that distinguish this from the formation of vertebrae in tetrapods and cartilaginous fish are: the role and persistence of the notochord which forms a central part of the vertebrae; the four distinct layers of bone of which the inner two form by mineralisation of a collagen matrix, and the outer two layers form by direct ossification (directly as bone), i.e. no part is preformed as cartilage; and there is no resegmentation.
Amphibians – frog
The amphibians (e.g. frog) present a notable variation on the way in which tetrapod vertebrae form. As with other tetrapods, the vertebra form from somite-derived sclerotome cells; but the somites form in a quite different way.[9] [10]
The radical differences are indicated in Figure 5 which is a composite – comparing what happens in an amphibian (Xenopus laevis) on the left with the usual tetrapod on the right.
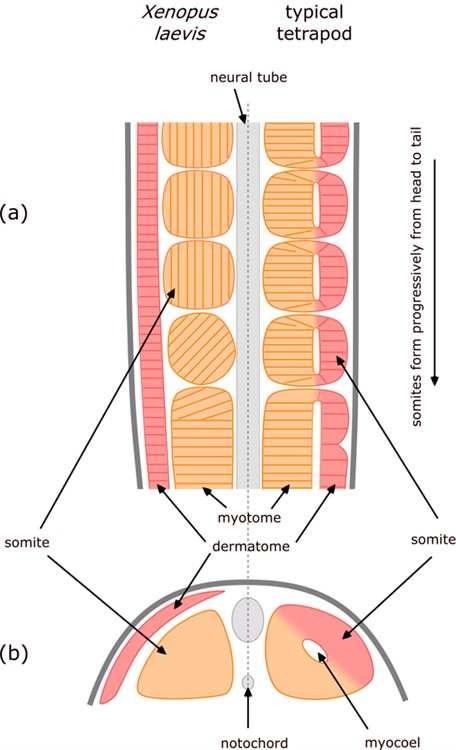
Figure 5. Comparison of somite formation in amphibian (Xenopus laevis) and typical tetrapod. Longitudinal section (a) showing progressive formation of somites, and cross-section (b) through newly-formed somites.
As the neural tube and notochord form, they are flanked by an unsegmented double layer of mesoderm. The inner layer of mesoderm (alongside the neural tube and/or notochord) is called myotome and the outer layer is called dermatome.
In most tetrapods, the somites form by lengths of these two layers pinching off – so the inner part of the somite is derived from myotome, the outer from dermatome, and the gap in between leads to the hollow centre of the somite, called a myocoel.
In contrast, in amphibians the somites form exclusively from the inner myotome layer, there is no myocoel, and the dermatome remains as a distinct layer on the outside of all of the somites.
Another interesting feature is that in amphibians, as cells of the myotome break off to form somites, the cells rotate through 90º so that they become orientated parallel to the neural tube / notochord – whereas in other vertebrates the cells remained aligned perpendicular to it.
Hamilton[7] sums up the situation as follows:
The presumptive somitic material of the early neurula and the fully differentiated axial musculature and dermis of Xenopus appear to be very similar to those of other vertebrates [which is why they are generally considered to be homologous]. Yet the intervening stages of morphogenesis are strikingly different [which refutes that presumed homology].
This is an important example: Some might argue (in view of their similar formation of vertebrae from somites) that at least tetrapods could have been derived from a common ancestor (even if different from the fish ancestors considered above). However, this significantly different formation of somites in amphibians (the first tetrapods) undermines the presumed common ancestry of tetrapods.
Why the evidence about homology is important – and hence ignored!
The fact that apparently homologous organs, especially key ones such as vertebrae, in fact have proved not to be, is extremely significant: it doesn’t just remove circumstantial evidence in support of evolution – it constitutes clear counter-evidence against the organisms concerned having evolved from e.g. a common vertebrate ancestor. This evidence unequivocally refutes macroevolution.
Needless to say there is profound reluctance within the scientific community to accept this conclusion, and the incongruence of embryology and presumed phylogeny has provoked earnest discussion among biologists over the last couple of decades to try to come to terms with the facts within an evolutionary framework. Unfortunately I do not have the space here to describe the responses as fully as I’d wish;[11] in summary:
1. Many assume that the different developmental routes to ‘homologous’ organs demonstrate that developmental processes can evolve while retaining the adult structure. But there are several objections to this:
-
It flies in the face of the evidence (and common sense) that tinkering with early development has seriously detrimental (usually fatal) consequences, rather than producing anything useful.
-
As said above, the case against this happening is strengthened the more we learn about the genetic and molecular mechanisms of embryological development. Given the large number of simultaneous coordinated changes that would be required, it beggars belief that such complementary mutations could happen opportunistically.
-
Indeed, what rationale is there to account for such a change? What advantage (that natural selection could work on) could drive radical changes in embryological development to comparable adult forms, given that natural selection acts primarily on the adult?
-
Taking the preceding two points together: there must be a viable evolutionary route – by random yet advantageous small steps.
-
It is no good simply saying that despite the theoretical objections the evidence shows that it must happen, because that’s a circular argument: rather than accept that different embryological development shows that the organisms did not have a common ancestry, it is assumed that common ancestry is true, so embryological processes must have changed, no matter how unlikely that is.
2. Convergent evolution. Some assume that the very similar organs have evolved independently, i.e. they are not homologous but what is called homoplastic. Quite apart from the extreme improbability of this, even if it were true, the non-homologous development still shows that the organisms in question did not share a common ancestor.
3. Revert to a pre-Darwinian view of homology. That is, some argue that homology merely describes features that are similar in various ways, but not necessarily with comparable embryological development. Again, those adopting this position must recognise that it not only removes homology as support for evolution, but it also does not get rid of the evidence that non-homologous development of so-called ‘homologous’ structures is not consistent with common ancestry.
Teach the controversy? – at least teach the facts!
4. Perhaps the response that is of most concern is that of suppressing the evidence.
The inconsistent embryological development of so-called ‘homologous’ organs has been known for a long time (e.g. Darwin (1866)[12], Spemann (1915)[13], de Beer (1971)[14]).
Yet what do we find in evolutionary textbooks? Almost exclusively they present the examples of homology that are perceived as consistent with evolution (notably the tetrapod skeleton) and little if any mention of the inconsistencies.
For example, Berkeley University has part of its website devoted to evolution (evolution.berkeley.edu) which includes an ‘in-depth course on the science of evolution’. This includes homology as one of the ‘evidences for evolution’ and gives tetrapod limbs as an example. There is no mention whatever that any ‘homologous’ organs are formed by non-homologous embryological processes, not even in the section ‘The big issues’ which ostensibly identifies the outstanding problems with the theory of evolution. Indeed this section is introduced by the grossly misleading sentence that ‘All available evidence supports the central conclusions of evolutionary theory, that life on Earth has evolved and that species share common ancestors.’
This is typical of what our biology students (and wider public) are being taught. And no doubt is why so few, including professional biologists, know about the anomalies – they are kept safely out of sight so as not to upset the applecart.
It really is time the scientific community were at least honest about all the scientific facts relevant to evolution.
References
[1] Owen, Richard; Report on the Archaetypes and Homologies of the Vertebrate Skeleton, Report of the British Association for the Advancement of Science for 1846, 169-340 (1847). Includes (p175) his definition of a Homologue: The same organ in different animals under every variety of form and function.
[2] Shorter Oxford English Dictionary.
[3] Roth, L; On homology; Biolog. J. Linnean Soc., 22:13-29 (1984); emphasis added.
[4] Indeed, it’s because it is recognised that embryological development cannot change readily, that embryological processes (ontology) are often used as a basis for assigning evolutionary relationships (phylogeny).
[5] For other examples see Evolution under the microscope.
[6] A useful overview can be found in Kardong, Kenneth; Vertebrates: Comparative Anatomy, Function, Evolution, 5th Edition (2008); Chapter 8: Skeletal system: The axial skeleton.
[7] Williams, E. Gadow's Arcualia and the Development of Tetrapod Vertebrae. The Quarterly Review of Biology, Vol. 34, No. 1 (Mar., 1959), pp. 1-32, at p19.
[8] Nordvick, Kari, et al.; The salmon vertebral body develops through mineralisation of two preformed tissues that are encompassed by two layers of bone; J. Anat. 206: 103-114 (2005).
[9] Hamilton, L; The formation of somites in Xenopus, J. Embryol. exp. Morph. 22(2): 253-64 (1969).
[10] Sparrow, D; Old Wares and New: Five decades of investigation of somitogenesis in Xenopus laevis. In Somitogenesis, eds Maroto and Whittock, Landes Bioscience and Springer+Business Media (2008).
[11] A good summary can be found in Laubichler, M; Homology in Development and the Development of the Homology Concept; Amer. Zool. 40: 777-788 (2000).
[12] Darwin, C; On the Origin of Species, 4th Edition, Chapter 13 (1866).
[13] Spemann, H; Zur Geschichte und Kritik des Begriffs der Homologie (A history and critique of the homology concept). In C. Chun and W Johannsen (eds.), Allgemeine Biologie. B. G. Teubner, Leipzig und Berlin. 3.4.1:63-85 (1915).
[14] de Beer, G; Homology: an unsolved problem; Oxford University Press (1971).
Image credits:
Thumbnail - abhijith3747 ©Fotolia images #53890339
Article images - all © the author